Rapidly Establishing an Ultra-Cold Supply Chain of Vaccines in Israel: Evidence for the Efficacy of Inoculation to Mitigate the COVID-19 Pandemic
Written by: Michael Naor, Gavriel David Pinto, Pini Davidov and Lina Abdrbo
The pandemic transformed old-fashioned pharmaceutical supply-chain networks because of the need to overcome the traditional hurdles of red tape to enable a fast response, which was essential as the public was in a state of panic. According to the World Health Organization, an ultra-cold chain (UCC) is needed for COVID-19 vaccines because of their requirement to be stored at ultra-low temperatures (ULT) of between −80 ◦C and −60 ◦C. While the pharmaceutical industry has an abundance of experience with the pipelines of common vaccines, the distribution of an ultra-cold supply-chain introduces an array of new challenges, such as the need for specialized packaging, shipment, and warehouses coupled with enhanced tracking for the handling of materials and border administration between countries. Because low-to-middle income countries do not have UCC capability, on behalf of COVID−19 Vaccines Global Access (COVAX), UNICEF acquired freezers which can store up to 300,000 doses each.
Israel rolled out its vaccination project for the emerging pandemic on 20 December 2020. The Israeli government attempted to secure contracts to procure the vaccine from global pharmaceutical companies because an Israeli-developed vaccine, called BriLife, was, in 2020, in the first clinical trial stage. By the time the United States Food and Drug Administration (FDA) issued an emergency use approval for the Pfizer-BioNTech COVID-19 vaccine, Israel had reached an agreement with Pfizer to purchase and receive millions of doses that would cover its full population.
The ulterior motive behind Pfizer’s decision to sign the contract stemmed from the fact that under Israeli law, all residents are insured with one of the four publicly funded health organization, all of which are managed by a centralized system containing the digital medical records of the entire country’s population. The entire operation of shipping the vaccines overseas from the United States to Israel was managed and supervised by a branch of the Israel Defense Forces titled the Israel Home Front Command.
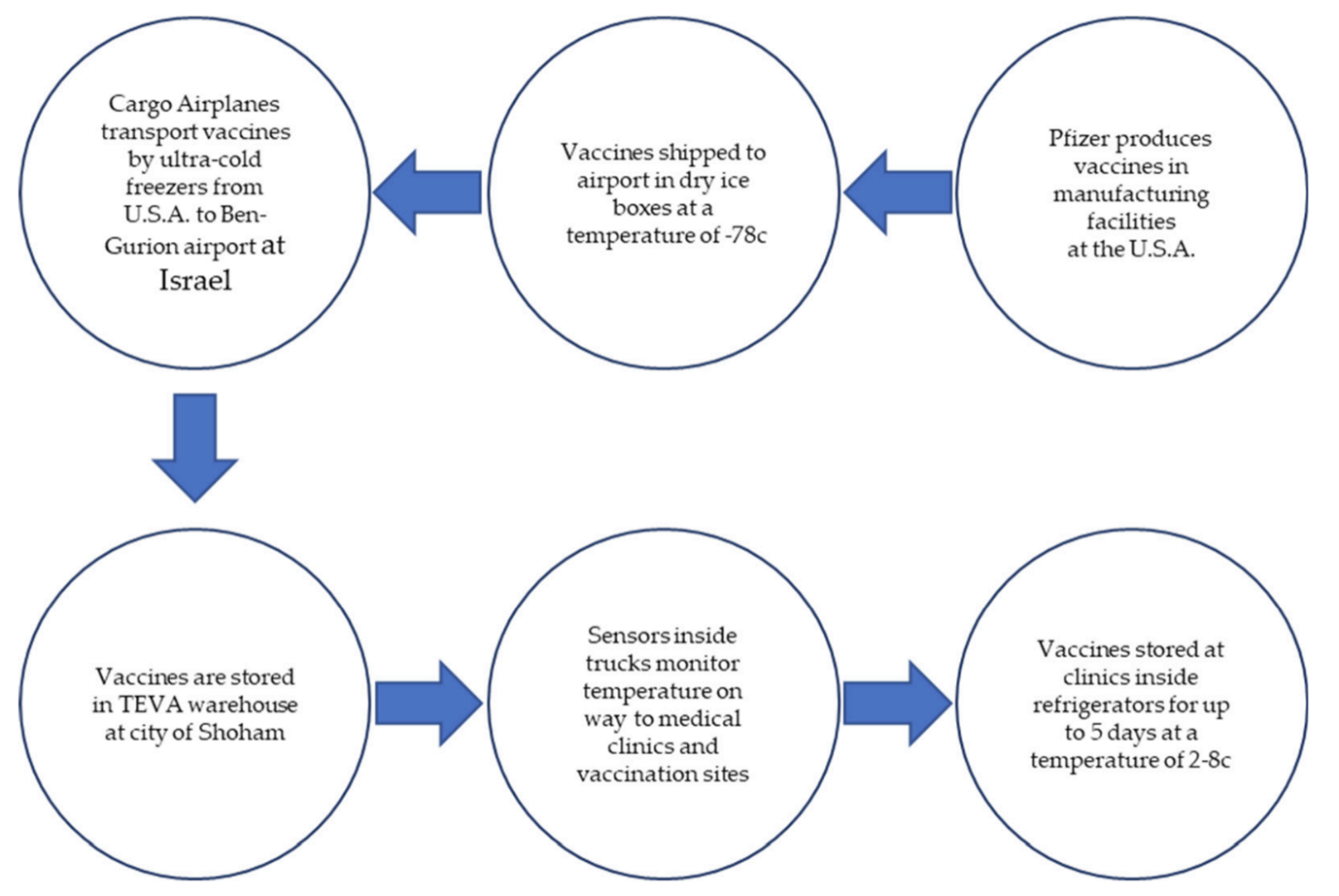
The Pfizer vaccines were shipped to Israel via air transport in specially designed, temperature-controlled containers utilizing dry ice to maintain the recommended storage temperature conditions of approximately −70 ◦C. The thermally monitored containers were then transported by truck from the airport to a state-of-the-art technology logistics warehouse operated by the SLE Ltd. pharmaceutical supply chain conglomerate in the city of Shoham, where the vaccines were stored in freezers.
Specifically, our study’s goal was to investigate two research questions:
(1) how can a country rapidly establish an ultra-cold supply chain for vaccines? (2) What was the impact of the coronavirus vaccine doses on Israel’s morbidity indices?
The method of the research combines interviews with seniors who were responsible on part of the supply-chain and analysis of a dataset publicly available from the Israel Ministry of Health regarding the daily rates of people vaccinated, tested and hospitalized.
The result shows that the successful Israeli paradigm of emergency response can be used as a template for countries interested in following in Israel’s footprints for creating a rapid process to establish an ultra-cold supply chain for vaccines before new variants emerge. While the ability to access patients’ records that are registered at various healthcare providers which do not pool medical information has been a bottleneck in the effort to coordinate a testing and vaccination campaign worldwide, Israel’s centralized healthcare database for the entire population has proven to be efficient at tracking healthcare data about the pandemic spread and the pace of vaccination. This may require governmental regulatory action to institutionalize laws allowing for the future sharing of information in countries where there is a large spectrum of public and private medical insurance providers.